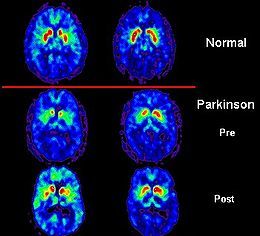
 PET scans showing dopamine activity in a normal brain and a Parkinson’s patient’s before and after treatment with a therapeutic implant.
PET scans showing dopamine activity in a normal brain and a Parkinson’s patient’s before and after treatment with a therapeutic implant.
More than a million Americans have Parkinson’s disease, and another 50,000 are diagnosed each year. Scientists know that many of the characteristic symptoms of Parkinson’s – tremors, rigid muscles and movement problems – can be traced back to the loss of dopamine-producing brain cells.
But what researchers don’t fully understand is why these cells are damaged in the first place. There are no clear environmental triggers for Parkinson’s, and in most people with the disease genetics doesn’t seem to play a large role either. It could even be that Parkinson’s is really a number of different diseases, all of which lead to the same end result.
Previous research has ruled out genetics as a major contributor to Parkinson’s risk; it is estimated to play a role in fewer than 10% of cases. But piecing together the genetics of Parkinson’s is an active and promising field, because what scientists learn from that minority of cases could improve their understanding of the disease generally. The holy grail would be to pin down all the ways genes can interact with the environment to produce the symptoms of Parkinson’s.
23andMe is working with the Michael J. Fox Foundation, the Parkinson’s Institute and patients from around the world partly because we want to give individuals with Parkinson’s access to their own genetic data. At the same time, we want to join the continuing quest for those genetic and environmental factors that underlie Parkinson’s disease by combining genetic data from our community with members’ responses to surveys about their health.
By far the most common known genetic cause of Parkinson’s disease is the G2019S mutation, which occurs in a gene called LRRK2. While the average person has a 1-2% chance of developing Parkinson’s, the risk for someone with the G2019S mutation is much higher and increases with age. One recent study found that a person who inherits this mutation from either parent has a 28% chance of developing Parkinson’s by the age of 59, 51% by the age of 69 and 74% by the age of 79.
Even though it is the most common known genetic cause of Parkinson’s disease, the G2019S mutation is responsible for only a small fraction of all cases worldwide – about one or two in a hundred. But in some ethnic groups it is a much more frequent cause of the condition. According to published research, up to 40% of people of North African Arab ancestry and 20% of Ashkenazi Jewish people with Parkinson’s have this mutation.
Another mutation in the LRRK2 gene, referred to as G2385R, has been associated with Parkinson’s disease in several East Asian populations. Analyses of the combined results from several studies indicate that people who inherit this mutation from either parent are at two to three times greater odds of developing Parkinson’s than those who do not carry the mutation. Approximately 9% of Parkinson’s patients with Asian ancestry carry the G2385R mutation.
(23andMe customers can find out if they carry the LRRK2 G2019S mutation in the Parkinson’s Disease Clinical Report. Customers can also find out if they carry the G2385R mutation using the Parkinson’s Disease: Preliminary Research Report.)
Although people who have at least one copy of the LRRK2 G2019S or G2385R mutation have an increased risk of Parkinson’s disease, a substantial proportion of people with these mutations never develop the disease. Very little is known about the function of the protein made by the LRRK2 gene, and no one knows why many people who carry mutations in this gene don’t develop Parkinson’s disease. Adding to the mystery, among those with these mutations who do develop Parkinson’s, not all have the same symptoms. Interactions of these mutations with unknown environmental factors may be part of the explanation. Continuing research into these gene-environment interactions could yield valuable clues into the causes of Parkinson’s generally.
Mutations in several other genes have also been associated with Parkinson’s disease, but these are extremely rare. Many have been found only in one or two families. 23andMe is not able to detect most of these rare mutations.
Other Parkinson’s mutations:
- As is the case for mutations in LRRK2, a person need only inherit a mutation in the alpha-synuclein gene (SNCA, also known as PARK1 or PARK4) from one parent to be at substantial risk for Parkinson’s disease. The average age for disease onset in people with a disease-causing mutation in this gene is 46. The protein encoded by the alpha-synuclein gene is one of the main components of the protein aggregates found in brain cells of people with Parkinson’s disease.
- Mutations in parkin (PARK2), PINK1 (PARK6) and DJ1 (PARK7) are recessive, which means a person must inherit mutated copies of these genes from both parents in order to develop Parkinson’s (there are rare exceptions). Multiple mutations in each of these genes have been identified; some are extremely rare.
- Parkinson’s disease caused by mutations in parkin, PINK1 or DJ1 usually strikes at a young age. Parkin mutations most often lead to disease onset between the ages of 20 and 40, although some people are younger than 10 or older than 60 when they develop symptoms. PINK1 mutations usually cause Parkinson’s between the ages of 32 and 48. DJ1 mutations are associated with an age of onset between 20 and 40 years of age.
This is an exciting time in the history of Parkinson’s research. The genetic aspects of the disease could reveal information that is invaluable in the ongoing search for new treatments, or even a cure. We invite Parkinson’s patients, their families and friends, and anyone who believes in the power of scientific research to change lives to participate in the 23andMe Parkinson’s disease community. Visit the 23andMe Parkinson’s community page to learn more.