Last month 23andMe received the first-ever FDA authorization for a direct-to-consumer genetic health risk test for cancer.
Eligible 23andMe Health + Ancestry Service customers now have the option to access their BRCA1/BRCA2 (Selected Variants) report.* The report looks at three variants in the BRCA1 and BRCA2 genes associated with an increased risk of breast, ovarian and prostate cancer. These three variants are most common in people of Ashkenazi Jewish descent, but having one of these variants is associated with increased risk no matter your ancestry.
To help customers understand the broader context around this report, we created a helpful webpage called Do You Speak BRCA?. This page looks not just at our report and customer stories, but includes information about the BRCA genes, and some of the history around research into BRCA-related cancers.
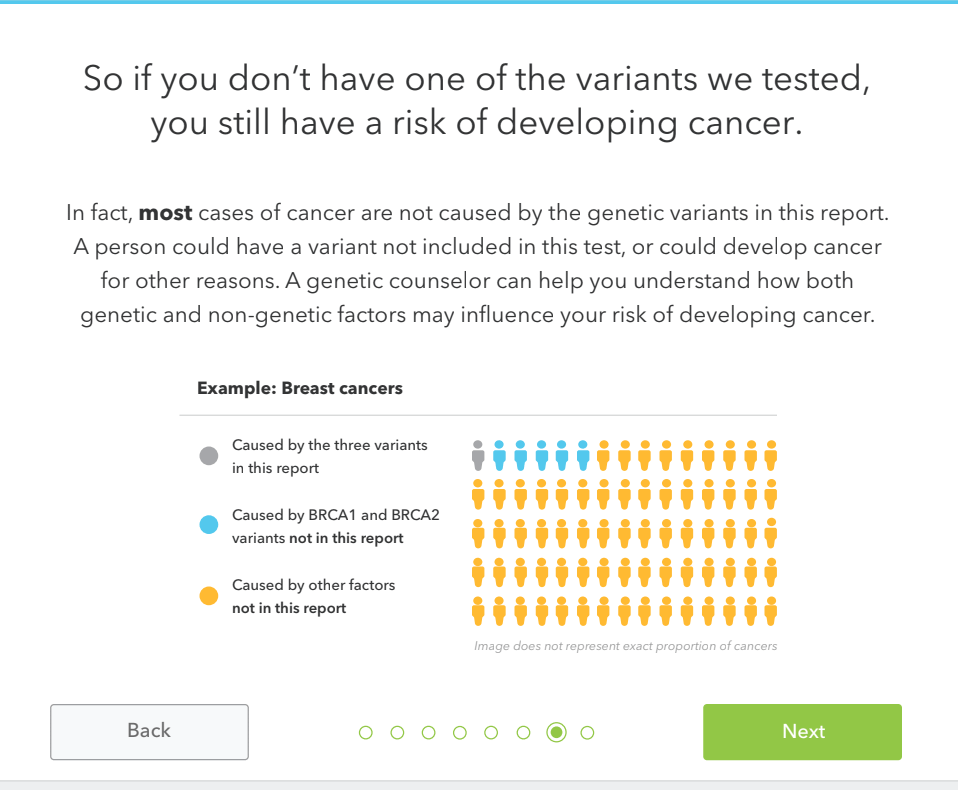
 If you choose to access your report, it’s important to understand the limitations. If your report shows no variants detected, it does not mean that you have no risk of cancer. 23andMe’s report looks at just three of the more than a thousand variants associated with an increased risk for breast, ovarian and prostate cancer, so you still could have another BRCA variant that 23andMe does not test for. In addition, most cases of cancer are not caused by inherited genetic variants, but by a combination of factors including environment and lifestyle. Another important limitation is that 23andMe’s test is not intended for diagnostic purposes, it’s important that you confirm your result in a clinical setting and talk to a healthcare professional before taking any medical action.
If you choose to access your report, it’s important to understand the limitations. If your report shows no variants detected, it does not mean that you have no risk of cancer. 23andMe’s report looks at just three of the more than a thousand variants associated with an increased risk for breast, ovarian and prostate cancer, so you still could have another BRCA variant that 23andMe does not test for. In addition, most cases of cancer are not caused by inherited genetic variants, but by a combination of factors including environment and lifestyle. Another important limitation is that 23andMe’s test is not intended for diagnostic purposes, it’s important that you confirm your result in a clinical setting and talk to a healthcare professional before taking any medical action.
Confirmatory testing is important, but rest-assured that the results provided in 23andMe’s BRCA1/BRCA2 (Selected Variants) report are highly accurate and meet FDA requirements for analytical, clinical and scientific validity. As with our other FDA-reviewed reports, each variant we report demonstrated greater than 99 percent agreement with an accepted comparison method. It also showed greater than 99 percent reproducibility and repeatability. Keep in mind that because this is an at-home test it is important to confirm results in a clinical setting before taking any medical action. Learn more.
Opting-In
Before customers can see their report, they’ll have to opt-in.
This is the same process as the opt-in for 23andMe genetic health risk reports on Parkinson’s disease and Late-Onset Alzheimer’s disease. The first part of that process asks if you want to see health reports in general, and the second part is specific to the Parkinson’s Disease*, Late-Onset Alzheimer’s Disease*, and BRCA1/BRCA2 (Selected Variants) reports, which must be chosen individually.

Before selecting whether to view the report, you can click “Learn more” to see additional information about the report, including what the report does and does not cover, ethnicity considerations, what different results might mean, and how results should be used.
For customers who do choose to view their BRCA1/BRCA2 (Selected Variants) report, they’ll also be required to view an educational module to help them better understand the information they’ll be seeing in the report itself. This brief tutorial describes some background about cancer, how genetics can play a role, and other concepts that are important to understand when viewing your report.
 Report Overview
Report Overview
23andMe Health + Ancestry Service customers will find the BRCA1/BRCA2 (Selected Variants) report to look familiar since it’s similar to the other Genetic Health Risk reports 23andMe offers.
The report has three sections — Overview, Scientific Details, and Frequently Asked Questions. The first thing a customer will see is whether or not they have one of the three tested variants and what that means. Again, getting a “variants not detected” result does not mean your risk of cancer is reduced or eliminated. It simply means that you do not have one of the three variants 23andMe tests for.
Below the main result, the report provides additional explanation about the customer’s result, along with details about BRCA-related cancer risks, information about other factors that influence cancer risk, and next steps that customers can take, including talking with a healthcare professional such as a doctor or genetic counselor.
.
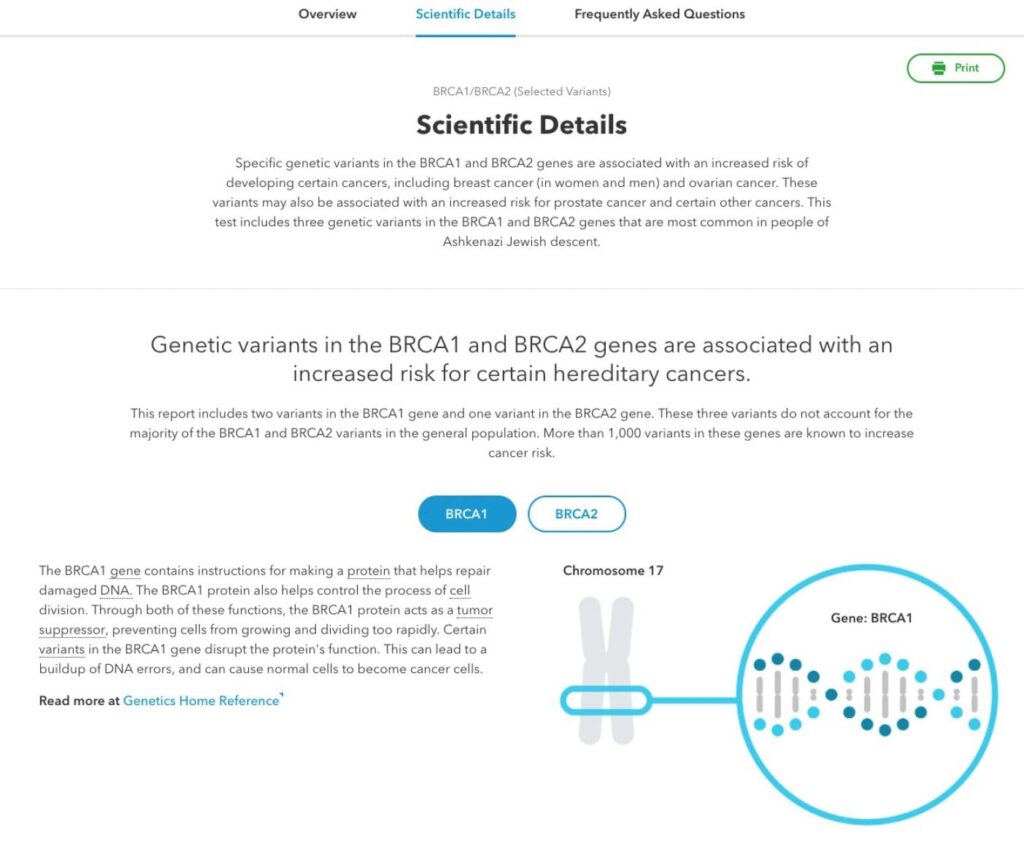
The Scientific Details
The Scientific Details page of the report provides more detail about the science behind the report. This includes information about the function of the BRCA1 and BRCA2 genes, how the three variants 23andMe tests for affect gene function, and risk estimates associated with having a BRCA variant. There also are more details about other factors that can influence cancer risk, including family history, lifestyle factors and genetic variants not included in our test. In addition, the report provides some information and resources about cancer screening.
 Further down you’ll see some technical details about the use and performance of the test.
Further down you’ll see some technical details about the use and performance of the test.
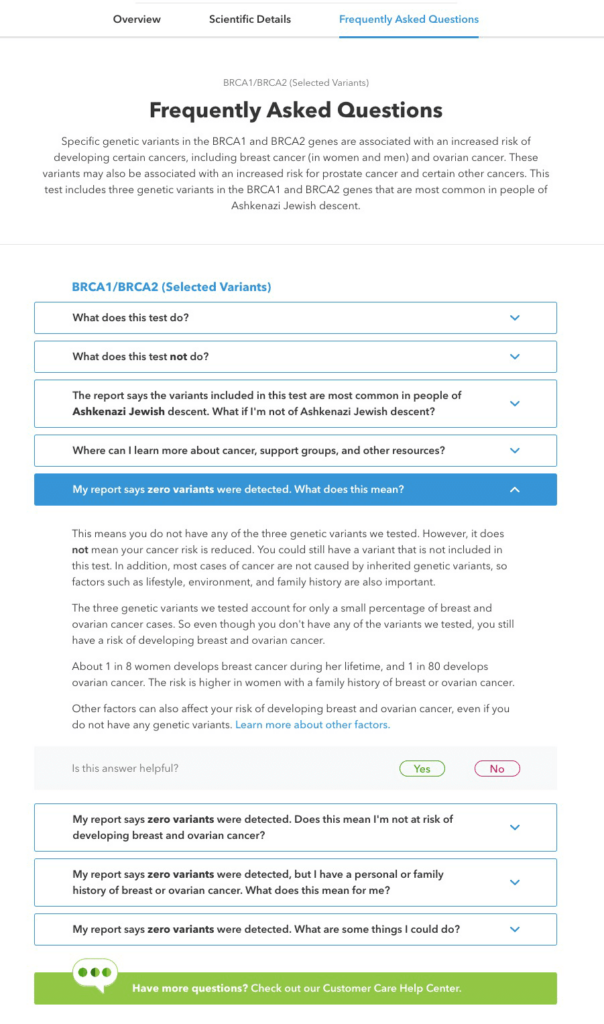
Frequently Asked Questions
In this section, we address some common questions customers may have about their report. Some of these are basic, like “What does this test do?” Others try to address issues like whether this report is relevant for people of different ethnicities or what to do if you have a certain result.
 For customers with a variant detected, we emphasize the importance of talking with a healthcare professional and confirming the result in a clinical setting. We also link to an article that walks through some of the considerations and conversations someone might have about their result, and highlights specific organizations that can further help customers navigate their care journey.
For customers with a variant detected, we emphasize the importance of talking with a healthcare professional and confirming the result in a clinical setting. We also link to an article that walks through some of the considerations and conversations someone might have about their result, and highlights specific organizations that can further help customers navigate their care journey.
The launch of the BRCA1/BRCA2 (Selected Variants) report is another milestone in our mission to help people access, understand and benefit from the human genome. We are excited to be able to offer this report to our Health + Ancestry customers.