FDA Now Recommends Genetic Testing Before Prescribing Specific Chemotherapy Medications
For years, patient advocates have been calling for stronger safeguards against preventable toxicity from chemotherapy. Last week, the FDA took a major step forward, updating the label for a drug called capecitabine to explicitly recommend testing for variants in the DPYD gene before starting treatment. This is more than just a label change—it’s a long-awaited acknowledgment that genetics matter when it comes to patient safety.
What is DPYD?
The DPYD gene provides instructions for making an enzyme called DPD (dihydropyrimidine dehydrogenase), which helps the body break down certain chemotherapy drugs like capecitabine and 5-fluorouracil. These medications are commonly used to treat breast, colorectal, and other cancers. People with certain changes in this gene may not process these drugs properly, causing the drugs to build up in the body and, in some cases, leading to severe or even life-threatening reactions.

These reactions can include painful mouth sores, severe diarrhea, low white blood cell counts, and nerve problems. Up to 8% of people have a variant in the DPYD gene that affects how well they process these medications. Sadly, some patients with DPYD variants have died after receiving these drugs. Such experiences have fueled years of advocacy calling for genetic testing before treatment, not after.
A change in the recommendations
Until now, the FDA advised doctors they could “consider testing” patients before giving capecitabine or similar drugs. The new label goes much further, adding a warning that says, “serious adverse reactions or death may occur in patients with complete DPD deficiency” and telling doctors to test for DPYD variants before prescribing the medication. (Experts predict a similar change to the 5-fluorouracil drug label soon.) It’s a major win for patient safety and for the advocacy groups who have pushed tirelessly for this change. Organizations that have long advocated for universal DPYD testing hope this update will encourage hospitals and cancer centers across the U.S. to make pretreatment genetic testing standard care.
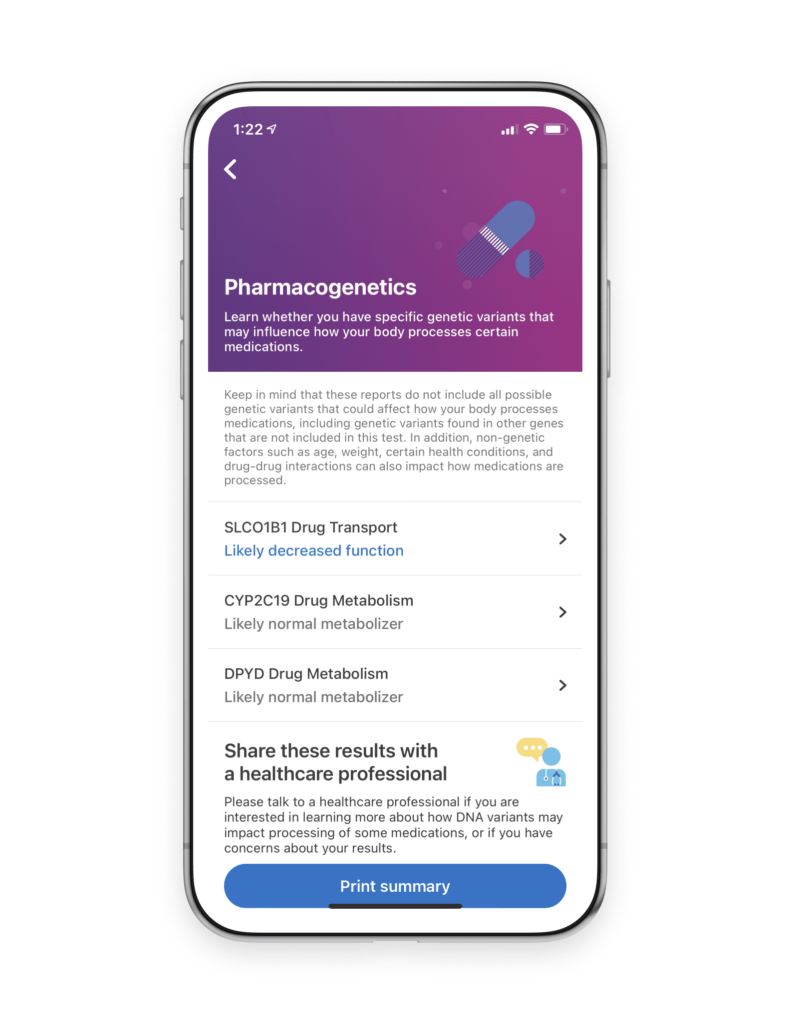
We share the belief that genetic insights can empower individuals and help improve outcomes. The 23andMe® DPYD Drug Metabolism Pharmacogenetics report*, available to 23andMe+ Premium™ members, provides information about two variants associated with reduced DPD enzyme activity. While this report isn’t a substitute for clinical testing, it can help people learn whether they carry certain DPYD variants and start a conversation with their healthcare provider.
The FDA’s new label is an important step toward safer, more personalized cancer treatment and a long-awaited victory for the patients and advocates who made their voices heard.
* 23andMe PGS Pharmacogenetics reports: The 23andMe test uses qualitative genotyping to detect 3 variants in the CYP2C19 gene, 2 variants in the DPYD gene and 1 variant in the SLCO1B1 gene in the genomic DNA of adults from saliva for the purpose of reporting and interpreting information about the processing of certain therapeutics to inform discussions with a healthcare professional. It does not describe if a person will or will not respond to a particular therapeutic. Our CYP2C19 Pharmacogenetics report provides certain information about variants associated with metabolism of some therapeutics and provides interpretive drug information regarding the potential effect of citalopram and clopidogrel therapy. Our SLCO1B1 Pharmacogenetics report provides certain information about variants associated with the processing of some therapeutics and provides interpretive drug information regarding the potential effect of simvastatin therapy. Our DPYD Pharmacogenetics report does not describe the association between detected variants and any specific therapeutic. Results for DPYD and certain CYP2C19 results should be confirmed by an independent genetic test prescribed by your own healthcare provider before taking any medical action. Warning: Test information should not be used to start, stop, or change any course of treatment and does not test for all possible variants that may affect metabolism or protein function. The PGS test is not a substitute for visits to a healthcare professional. Making changes to your current regimen can lead to harmful side effects or reduced intended benefits of your medication, therefore consult with your healthcare professional before taking any medical action. For important information and limitations regarding Pharmacogenetic reports, visit 23andme.com/test-info/pharmacogenetics/



